Data Integrity Sop in Pharmaceutical Industry
Web The ALCOA acronym is a concept of data integrity based on the accurate complete and consistent recording and management of a data or information either on paper or electronically. In its broadest use data integrity refers to the accuracy reliability and consistency of data stored over its entire life-cycle in a database data warehouse data mart or other construct.

Sop On Data Integrity In Pharmaceutical Industry
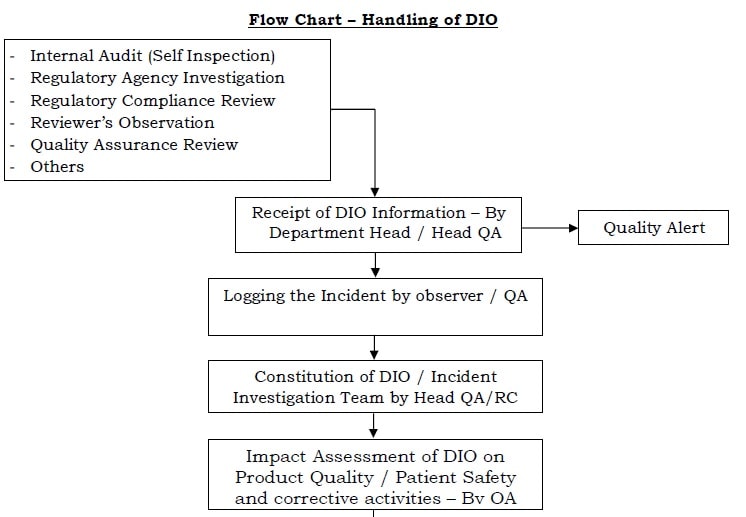
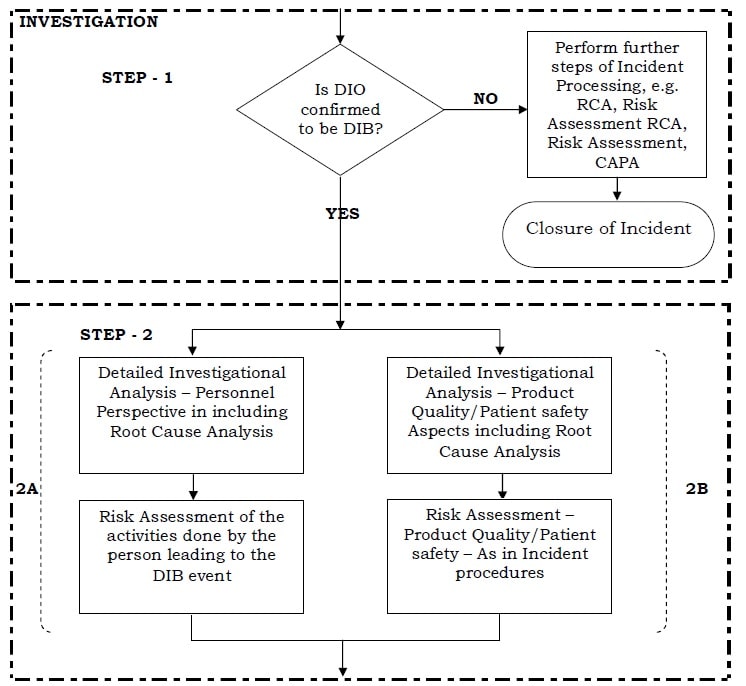
To outlines the process for Handling of Data Integrity Observations DIO in order to ensure that each such observation is identified documented investigated and concluded appropriately along with with implementation of any corrective andor preventive actions.
. Information Technology 63 QA. The purpose of good documentation practices procedure to maintain data integrity. Web The FDAs expectation from the pharma industry is data supporting pharmaceutical product registrations are reliable and accurate.
Systems to assure data quality and integritysystems should be designed in a way that encourages compliance with the principles of data integrity for examples include -. Web These days pharmaceuticals have started relying on computers and automated systems to a great extent whether in terms of manufacturing laboratory release testing or many other tasks involved. Categories Free Engineering Course Free Manufacturing Course Free Quality Assurance Course.
All the data either in manual form or electronic must maintain its integrity. Web What is data integrity. For the purposes of this guidance data integrity refers to the completeness consistency and accuracy of data.
Web Up to 3 cash back This procedure emphasizes that everyone in the company is responsible for data integy. Issuance of a quality system policy ensuring the integrity of data unless the policy addresses all relevant aspects of the companys operations including personnel behaviors and actions. Web The Food and Drug Administration FDA has also published a new data integrity guidance aimed at assisting pharmaceutical manufacturers to meet the regulators standards.
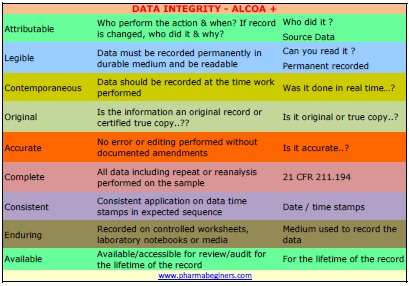
The purpose of this Standard Operating Procedure SOP is. The US-FDA uses the term ALCOA which refers to complete. The generated or collected data must.
It is the responsibility of the pharmaceutical industry to ensure the efficacy. Which relates to the agenda of Good Manufacturing Practice GMP-related concerns. Web 525 Backup of data shall be taken periodically and the backup and recovery process shall be validated.
The term refers to the characteristics of data integrity which means that the data according to the FDA must be. Data must be completed and accurate without any alteration. Web Without any confirmation of vials in system according the sequence dont start the run.
This SOP is helpful to understand how the GDP plays an important role to minimize data integrity by following the ALCOA principle. This procedure is appicable to all manufacturing. Data Integrity shall be maintained in all manual or system generated electronic data.
The term Data Integrity can be used to describe a state a process or a. 16 The purpose of this guidance is to clarify the role of data integrity in current good manufacturing 17 practice CGMP for drugs as. Web Data integrity is a fundamental component of information security.
Data integrity DI ensures that the data generated during business operations and drug manufacturing is accurate complete and reliable. Web Across the pharmaceutical industry this means that new frameworks of actions need to be adopted into business operations to reap the benefits associated with these technologies. Firms should implement meaningful and effective.
In the pharmaceutical industry ensuring data integrity involves generating and documenting data accurately protecting data from accidental or intentional modifications falsification deletion or destroying data. Web Subsequently inferior quality products may be unsafe for patients. Data integrity breaches can result from poor practices or inadequate systemsprocedures.
Any issue in data integrity must be handled as per the quality management system QMS and proper corrective and preventive action CAPA must be taken according to risk assessment. Web 10 PURPOSE. Any identified data integrity issue shall be handled as per the quality management system and proper corrective and preventive action shall be taken according to risk assessment.
Complete consistent and accurate data should be attributable legible contemporaneously recorded original or a true copy and accurate ALCOA. 526 Proper training on data integrity and usages of the computer systems shall be provided to all concerned personnel 60 ABBREVIATIONS 61 SOP. Web SOP on Data Integrity in Pharmaceutical Industry.
Seamlessly connect incubation detection enumeration to uphold ALCOA data integrity. According to FDA data integrity refers to the completeness consistency and accuracy of data. Ad Uphold quality control data integrity by reducing human error.
Web All pharmaceutical industry follows the good documentation practices for the consistency in documentation. The procedure includes reporting and investigation of breach of data Integy and training and education of employees for ongoing compliance wih the requirements of data integtiy. Ad Proven System to Create Manage SOPs Operations Manuals.
SOP on Data Integrity in Pharmaceutical Industry Part 1 0442 Part 2 0450 Student Ratings Reviews 45 Total 4. 3 4 Only when. Standard Operating Procedure 62 IT.
Access to clocks for recording timed events. Data should be complete and accurate without any alteration. A quote from the guidance is cGMP regulations and guidance allow for flexible and risk-based strategies to prevent and detect data integrity issues.
Action must be. Due to this a renewed focus has been brought to the concept of data integrity.
Data Integrity Handling Of Di Observations Dio Pharma Beginners

Data Integrity Handling Of Di Observations Dio Pharma Beginners
Data Integrity Handling Of Di Observations Dio Pharma Beginners

0 Response to "Data Integrity Sop in Pharmaceutical Industry"
Post a Comment